
Insights+: EMA Marketing Authorization of New Drugs in January 2024
Shots:
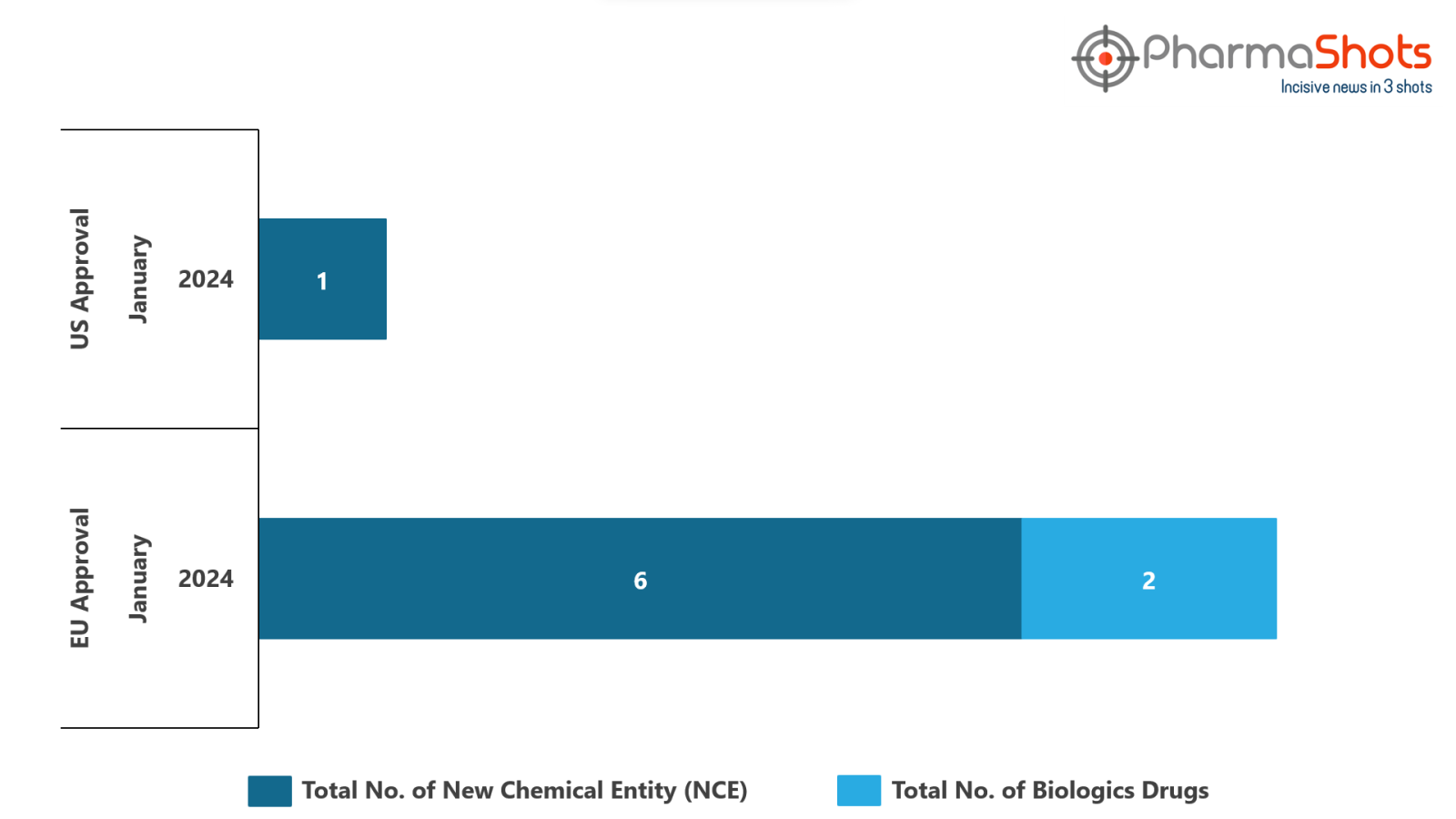
- The EMA approved 2 BLA and 6 New Chemical Entities in January 2024, leading to treatments for patients and advances in the healthcare industry
- In January 2024, the major highlighted drugs were Padcev to treat Bladder Cancer and Eylea for the treatment of Age-related Macular Degeneration (nAMD) and Diabetic Macular Edema (DME)
- PharmaShots has compiled a list of a total of 7 new drugs approved by the EMA in January 2024
Product Name: Padcev + Keytruda
Active ingredient: Enfortumab Vedotin + Pembrolizumab
Company: Astellas
Date: Jan 29, 2024
Disease: Bladder Cancer
- The application was submitted based on the results from the P-III (EV-302/KEYNOTE-A39) clinical trial evaluating Padcev + Keytruda vs platinum-containing CT (SoC) in patients (n=886) with previously untreated la/mUC. The 1EPs of the study were OS & PFS whereas the 2EPs were ORR, DoR & safety
- The results depicted that the study met its dual 1EPs with a mOS of 31.5mos. vs 16.1mos. (reducing the risk of death by 53%) & mPFS of 12.5mos. vs 6.3mos. (reducing the risk of death by 55%). The results were presented at ESMO 2023
- Padcev is an ADC developed to function against Nectin-4, the protein located on the surface of cells expressed in bladder cancer. The combination of Padcev + Keytruda was approved by the US FDA in Dec 2023
Product Name: Hyqvia
Active ingredient: Immune Globulin Infusion with Recombinant Human Hyaluronidase
Company: Takeda
Date: Jan 29, 2024
Disease: Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
- Takeda’s Hyqvia has been approved as maintenance therapy for treating chronic inflammatory demyelinating polyneuropathy (CIDP) on stable IVIG therapy valid throughout the EU plus Iceland, Liechtenstein, Norway & Northern Ireland
- The approval is based on the results from the P-III (ADVANCE-CIDP 1) study assessing Hyqvia’s safety and efficacy as a maintenance therapy to prevent relapse in CIDP patients (n=132) on stable IVIG therapy
- The results revealed reduced CIDP relapse rate of 15.5% with Hyqvia vs 31.7% in PBO with a treatment difference of -16.2 favoring the drug
Product Name: Abecma
Active ingredient: Idecabtagene Vicleucel
Company: Bristol Myers Squibb
Date: Jan 26, 2024
Disease: Multiple Myeloma
- The CHMP positive opinion was based on the results from the P-III (KarMMa-3) clinical trial evaluating Abecma vs standard regimens in patients with r/r multiple myeloma following 2-4 prior lines of therapy. The 1EP of the study was PFS & 2EPs were ORR & OS
- Following a median follow-up of 30.9mos., the study showed a PFS of 13.8mos. vs 4.4mos., depicting a 51% reduction in risk of disease progression with Abecma & an ORR of 71% vs 41% with CR in 44% vs 5%. The results were presented at the ASH Annual Meeting in Dec 2023
- Abecma is a CAR T cell therapy that functions by recognizing & binding to the surface of multiple myeloma cells & thereby causing CAR T cell proliferation, cytokine secretion & subsequent cytolytic killing of BCMA-expressing cells
Product Name: Ryzeneuta
Active ingredient: Efembalenograstim Alfa
Company: Evive Biotechnology
Date: Jan 25, 2024
- The CHMP has granted a positive opinion to Ryzeneuta (20mg, IV) based on its safety and efficacy evaluation results as compared to PBO
- The recommendation was made by CHMP for EC’s approval as medicinal product for the reduction in the duration of neutropenia and the incidence of febrile neutropenia in adult patients treated with cytotoxic chemotherapy for malignancy
- Efembalenograstim alfa is an immunostimulant/colony-stimulating factor belonging to the class of haematopoietic growth factors that functions by increasing the production and differentiation of mature and functionally active neutrophils from bone marrow precursor cells
Product Name: Rystiggo
Active ingredient: Rozanolixizumab
Company: UCB
Date: Jan 08, 2024
Disease: Generalized Myasthenia Gravis (gMG)
- The approval was supported by the P-III (MycarinG) study evaluating the safety, efficacy & tolerability of Rystiggo (7 or 10mg/kg) vs PBO in gMG patients. The 1EP of the study includes a change in MG-ADL score at Day43 & 2EPs include a change in MG-C score, QMG, patient-reported outcomes at Day43 and AEs
- The trial depicted clinically meaningful & statistically significant improvement in gMG-specific outcome vs PBO as reduction in MG-ADL scores from baseline to Day43 were greater with both doses vs PBO
- Rystiggo, humanized mAb that specifically binds to human FcRn, received the EU’s ODD in 2020 for gMG. The approval came following the US FDA & MHLW’s approval for Rystiggo & Zilbrysq & EC’s approval for UCB’s Zilbrysq in 2023
Product Name: Eylea
Active ingredient: Aflibercept
Company: Bayer and Regeneron
Date: Jan 08, 2024
Disease: Age-related Macular Degeneration (nAMD) and Diabetic Macular Edema (DME)
- The approval was granted based on the results from the P-III (PULSAR) & P-II/III (PHOTON) clinical trials evaluating the safety & efficacy of Eylea 8mg vs 2mg dosed Q8W following initial monthly dosing in patients (N=1,164) with nAMD & DME. The 1EP of both the studies was non-inferior BCVA changes
- Both the studies met their 1EP of non-inferior BCVA changes in patients receiving 8mg of Eylea for 12-16wks. dosing regimen vs those receiving 2mg for fixed 8wks. treatment interval at wk.48. Moreover, the safety profiles of the study were consistent with the previous studies of Eylea (2mg)
- Earlier in Aug 2023, the US FDA approved aflibercept (8mg) by the name Eylea HD for nAMD & DME while more applications for approval have been submitted by Bayer to other regulatory bodies. Eylea (8mg) is jointly being developed by Bayer & Regeneron
7. BMS Reports EMA’s Validation of Augtyro (repotrectinib) for the Treatment of Solid Tumors
Product Name: Augtyro
Active ingredient: Repotrectinib
Company: Bristol Myers Squibb
Date: Jan 02, 2024
Disease: Solid Tumors
- The EMA has validated MAA of Augtyro for treating adults and pediatric patients (12yrs. & older) with ROS1+ and NTRK+ locally advanced or metastatic solid tumors incl. NSCLC
- The application was based on two P-I/II (TRIDENT-1 & CARE) trials assessing the safety, tolerability, PK and anti-tumor activity in adults with ROS1+ NSCLC or NTRK+ solid tumors and pediatric patients (12yrs. & older) with NTRK+ locally advanced/metastatic solid tumors, respectively
- The result depicted that of the 79% of TKI-naïve & 38% of TKI-pretreated patients who responded to treatment, 6% & 5% depicted a CR whereas 73% & 32% experienced PR along with an mDOR of 34.1 & 14.8mos. Moreover, patients with measurable CNS metastases showed responses in 7 of 8 TKI-naïve & 5 of 12 TKI-pretreated in (n=56) patients with intracranial lesions at baseline
Note:
- According to the EMA’s January 2024 approval list, Novartis’ Spexotras was also approved; however, no PR was available
Related Posts: Insights+: EMA Marketing Authorization of New Drugs in December 2023
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














